Background: Sequencing studies have advanced our understanding of the genomic underpinnings of Multiple Myeloma (MM). While whole genome sequencing (WGS) has emerged as a comprehensive approach for profiling the genomic landscape of MM, WGS has limited sensitivity in defining the intrapatient genomic heterogeneity. Single cell RNA approaches can capture phenotypic heterogeneity but fail to recover most of the key genomic drivers. To overcome these historical limitations, we utilized a newer single cell whole genome direct library preparation (scWGS DLP+; Lacks et al. Cell 2019) sequencing technology able to detect early changes and study clonal population dynamics, providing a more precise understanding of the emergence and clinical impact of genomic events during MM evolution.
Methods: scWGS DLP+ was applied to CD138 + Plasma Cells (PC) isolated from bone marrow samples of MM patients. Using newly developed computational approaches, we characterized the Single Nucleotide Variants (SNVs), copy number changes and complex Structural Variants (SVs) at a single cell level and use this information to reconstruct the evolutionary histories of each patient's myeloma.
Results: We applied DLP to 7 MM patients obtaining 800-1600 single cell whole genomes per patient. All canonical IgH translocations identified by FISH were recovered in the scWGS data. Single cell copy number heterogeneity was observed in all but 1 patient, including heterogeneity affecting known drivers. The number of subclones ranged from 1 to 15, with an average of 6 subclones detected per patient (≥10 cells, ≥5MB distinct segmental difference). This is in striking contrast with bulk WGS where we are usually able to define no more than one or two subclones. Gain of 1q (3 copies) was observed in all patients but was either rare (1-3%, 3 patients) or clonal (>99%, 4 patients), with two clonal 1q patients exhibiting convergent subclonal 1q amplification (>3 copies). Loss of previously gained chromosomes was observed including loss of ancestrally gained chromosome 15 in hypodiploid Patient 2, and sporadic loss of 1q in patients with ubiquitous 1q gain. For 3 of 7 patients, hypodiploidy (HRD) had arisen before the most recent clonal expansion. HRD clones continued to diversify, accruing multiple additional events on HRD chromosomes including large or focal gains, or deletions subsequent to a gain. Interestingly, one case had 1 small hypodiploid clone co-existing with a completely different major clone with 1q and t(14;20) IgH translocation, suggesting the existence of two distinct clonal plasma cell populations (one malignant and one likely bening). Two of the 7 patients harbored both small (<20 cells) whole genome doubled (WGD) subclones, and small numbers of highly aneuploid likely produced by multi-polar cell division post WGD.
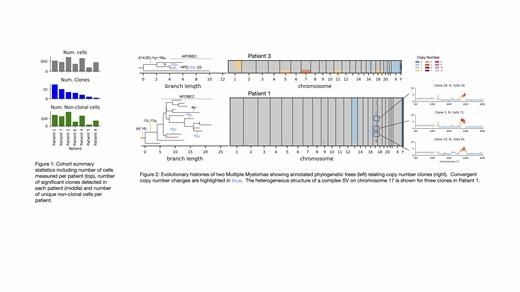
We explored two patient's evolutionary histories in detail, highlighting the unique resolution of scWGS DLP+ to detect CNV but also SNV, mutational signatures and SVs. Patient 3 exhibited 4 distinct subclones with distant evolutionary origin as evidenced by the relatively small number of shared SNV. APOBEC signature mutations were detected in all 4 subclones suggesting APOBEC mutagenesis was still ongoing across the cell populations in this patient. In Patient 1, a complex SV on chromosome 17 resulted in loss of most of 17q and an amplification of 17q segments. The heterogeneous structure of chromosome 17 across clones suggests a late onset of this complex event and ongoing instability. Chromosome 16 exhibited a pattern of cell specific arm loss likely resulting from telomere loss. Interestingly, a minor clone showed complex amplification of 16p affecting TNFRSF17, suggesting that chromosome 16 is at an earlier stage of a process of instability that starts with cell specific arm loss, and ends with clonal expansion of a complex amplification SV targeting a gene with fitness benefit.
Discussion: Applied to Multiple Myeloma, scWGS shows higher clonal heterogeneity than previously described with multiple clones exhibiting diverse genetic alterations. The findings underscore the genomic complexity and dynamic nature of MM, highlighting the need for sensitive techniques like scWGS to capture the full spectrum and chronological order of genomic events.
Disclosures
Landgren:Adaptive: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Theradex: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees. Usmani:Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; EdoPharma: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; K36 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Moderna: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; SkylineDX: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; TeneoBio: Membership on an entity's Board of Directors or advisory committees; Array Biopharma: Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding. Shah:Canesia Health Inc: Current equity holder in private company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal